10 Tips To Ensure Your GMP is a Quality Priority
10 Tips To Ensure Your GMP is a Quality Priority
10 Tips To Ensure Your GMP is a Quality Priority
.png)
As we are all too aware, maintaining a high level of product quality is a series of actions that endure throughout that product’s lifecycle. These actions are often at the core of a business optimization strategy, but quality must start with the manufacture of safe products that adhere to regulatory guidelines.
I recently participated in a recent quality audit for one of Orcanos’ customers. The audit itself was fairly standard but the auditor took some time to explain to me the tremendous amount of investment that small companies will have to make in quality processes in the near future.
Over the next two to five years, he said, a defined need to be compliant with evolving medical device regulations is going to put companies under a lot of pressure, with Good Manufacturing Practice (GMP) at the top of the list.
To say that this was mind-blowing is an understatement. I was driven to put my thoughts down in writing, and this blog post is the result.
With that in mind, I would like to share with you both the GMP elements that you need to be aware of and my tips for keeping GMP at the forefront of quality conversations.
On a very basic level, GMP establishes minimum standards for product manufacturing, with the aim being to prevent harm from occurring to the end user. In most cases, companies will use the guidelines to limit adulteration and ensure that a high level of quality is present in every product.
This should not be a surprise, but, importantly, GMP needs to be a so-called “lifestyle” that each company clearly defines and implements throughout its quality systems, with the safety of its customers an overarching priority.
Taking that into account, let’s take a look at how the integration of GMP into a workflow can be achieved.
There are 10 principles of good manufacturing principles that I believe can help in instilling a “GMP lifestyle” in your organization. These are as follows:
These 10 principles provide stakeholders with a framework for not only building and maintaining a GMP lifestyle but also help to evaluate how well a company is complying with the standards of good manufacturing practices.
So, let’s take a deeper dive into how these defined principles play out in the product and quality journey.
The first two GMP principles stress the importance of written procedures. In fact, the best way to comply with GMP regulations is to have well-written procedures and to carefully follow them.
These written procedures give us the controls necessary to minimize the chance of mix-ups and errors in manufacturing a product. When we carefully follow written procedures, we not only ensure compliance with the GMP regulations but also ensure the consistent quality of our products.
Principles 3 and 4 stress the need to document and validate your work. Because documentation and validation are so important to the company, let’s look at them more closely.
We should start by asking, what does documentation really mean in terms of an individual job performance?
On a very basic level, documentation requires a specific action on somebody’s part. In other words, the recording of each significant step someone performs as they perform a job task. This means that documentation must be produced promptly, be accurate, and, crucially in accordance with written procedures.
As important as documentation is, it merely shows that written procedures have been followed, carefully and (in theory) to the letter. What matters more is the validation of that documentation.
Validation is proactive proof that we can produce safe and effective products. Taking that into account, validation, requires a series of tests to assure that systems and processes do what we say they do. That means that workers must be sure the production processes consistently meet the specifications the company has established.
Therefore, validation gives meaning to the documented records being kept. It is validation that tells stakeholders that written procedures are correct, and that products are truly safe and effective.
GMP principles 5 and 6 focus on the design, construction, and maintenance of facilities and equipment.
A key concern is to avoid the possibility of contamination, mix-up, and errors in the workplace. For example, it is important to keep certain areas – the cafeteria, locker room and washrooms, for example – separated from the manufacturing area.
Where it is necessary to protect the integrity of products, there must be careful control of water, air, temperature and humidity. Housekeeping, sanitation and maintenance also work to defend against contamination, mix-ups and errors.
The seventh GMP principle under the spotlight states that the establishment of these practices requires competent people.
People who can do the job right, the first time and every time are a vital part of the quality process. That means it is a worker’s personal responsibility to develop, demonstrate and continuously improve his or her job competence.
In order to do any job well, people must be properly trained. This requirement is particularly true in the manufacturing and quality control areas. In fact, our company must have a formal training program, to ensure that each employee can competently perform assigned job responsibilities.
That requirement may sound simple, but competence in one area may not be replicated in another. Let’s not forget that the workplace is essentially staffed by the human element, a requirement that leads to directly to the eighth GMP principle, which focuses on cleanliness and the defense of products against contamination.
Contamination can be a powerful and dangerous enemy, and it takes on many different forms.
One of the most common is Particulate Contamination. This means that a product has been made impure by any particle that doesn’t belong there. For example, dust, lint, fibers and hair are all potential causes of this form of contamination. That is why people must be properly dressed to prevent contamination when working with materials, components and products.
Another form to be aware of is Microbial Contamination. This is caused by microscopic organisms, known as microbes. Microbes are living organisms that exist one everything in the environment that has not been sterilized, and they can include. fungus, mold, bacteria and viruses.
Cross Contamination is the third form to be aware of. This impurity occurs when traces of other materials’ components and products adulterate or mis-brand the products a company is currently manufacturing, packaging or testing.
While this may GMP principle seem obvious, it is critical that everybody practices good personal hygiene, and helps to keep a workplace clean by reporting any condition, equipment or practice in a plant that might be a potential source of Particulate, Microbial or Cross Contamination.
The ninth GMP principle focuses attention on the importance of building quality into products, by systematically controlling the components and product-related processes.
To see how GMP helps you build quality, let’s examine the critical areas where we must establish effective controls. These can be grouped into materials and components, the manufacturing process, packaging and labelling, testing and safety
Materials and Components
Materials and components present the first critical control challenge.
We must be sure all of our components and materials satisfy our quality standards. Upon receipt, they must be carefully examined for damage and contamination, properly identified and tagged. After this has been completed, they must, be stored in a quarantine area.
Where required by regulatory compliance, certain components and materials must be sampled and tested to ensure they meet established standards of identity, quality and purity. Only after approval is secured can they be released to manufacturing and used on a first-in-first-out basis – in other words, the first materials and components approved for release are the first to go to manufacturing.
Manufacturing Process
The second critical area to be controlled is the manufacturing process itself. To ensure quality and uniformity of each product, there will be master records onsite that outline the specifications and manufacturing procedures, the individual batch or history records (E-DHR) to help document conformance to the master record and written schedules and procedures for cleaning and maintaining the equipment.
To help employees operate in a controlled state, written work instructions are carefully followed, critical data is accurately collected, and manufacturing results are documented without delay.
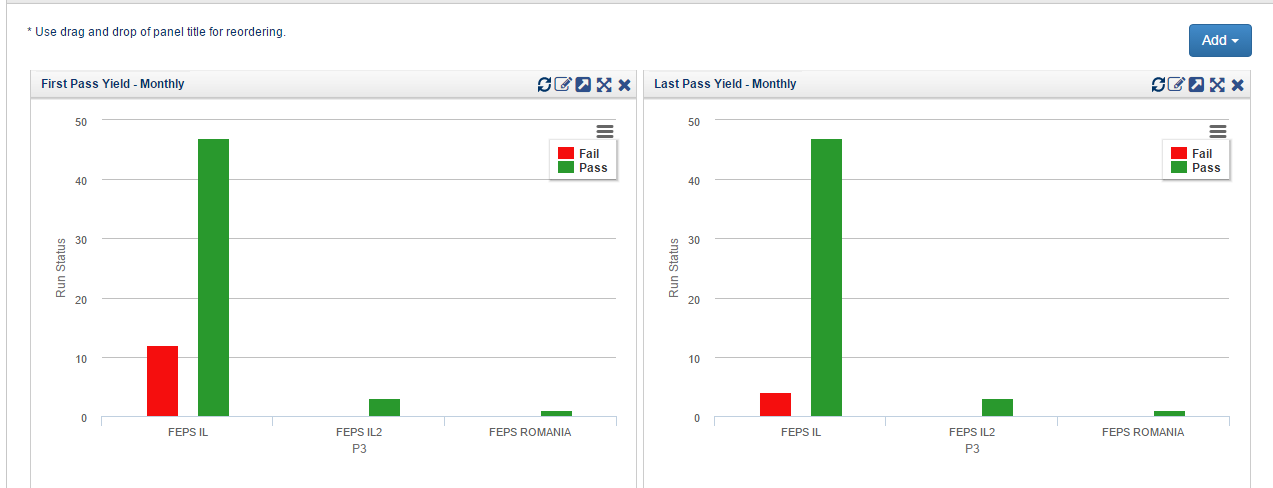
Fig 3: Production ATE Results Online Collection using Orcanos Rest API
Packaging and Labeling
A third critical area where quality control is required is packaging and labeling. There are literally thousands of examples of product recalls that can be traced back to errors in the labeling aspect, and it is an area that can sometimes slip under the radar.
With that in mind, the packaging and labeling area must be inspected before each new batch or lot is processed. This action helps to confirm that the packaging equipment is clean and that the area does not contain any materials from a previous run.
Testing
The fourth critical area is testing and supports all other areas of control. How we handle incoming, in-process and finished product test samples, how we perform test methods, how we document test results, are all significant elements of the testing process and must be performed by qualified individuals. All of these actions are a crucial step in the quality journey, and should be prioritized as such.
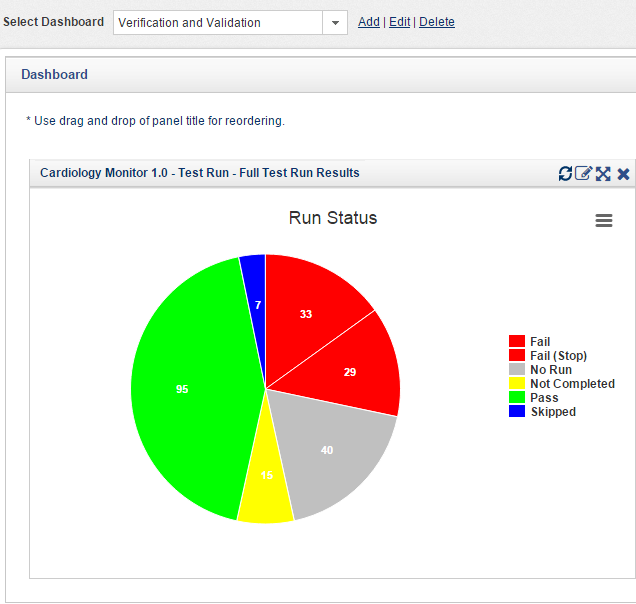
Fig 4: Test Protocols Results using Orcanos Test Management™
Finally, we need to focus on how to ensure the, safety, effectiveness and purity of the product, as it enters the marketplace.
The challenge is make sure that the quality ball is not dropped when the finished product is tested and released. Quality doesn’t stop after the sale is made, in some ways that should be the moment when vigilance is increased.
There must be careful and effective monitoring of the product within the warehouse and across the customer distribution ecosystem. Sales and marketing strategies should be assessed, with both the customer experience and successful campaigns used as benchmarks for future products.
These are the methods that successful brands use to interact with their customers, and we must keep accurate records to provide product traceability. In addition, there must be a prompt response to any customer problems, concerns or complaints. Brand reputation is aligned with the quality of the product, and it is customers that are often judge, jury and executioner.
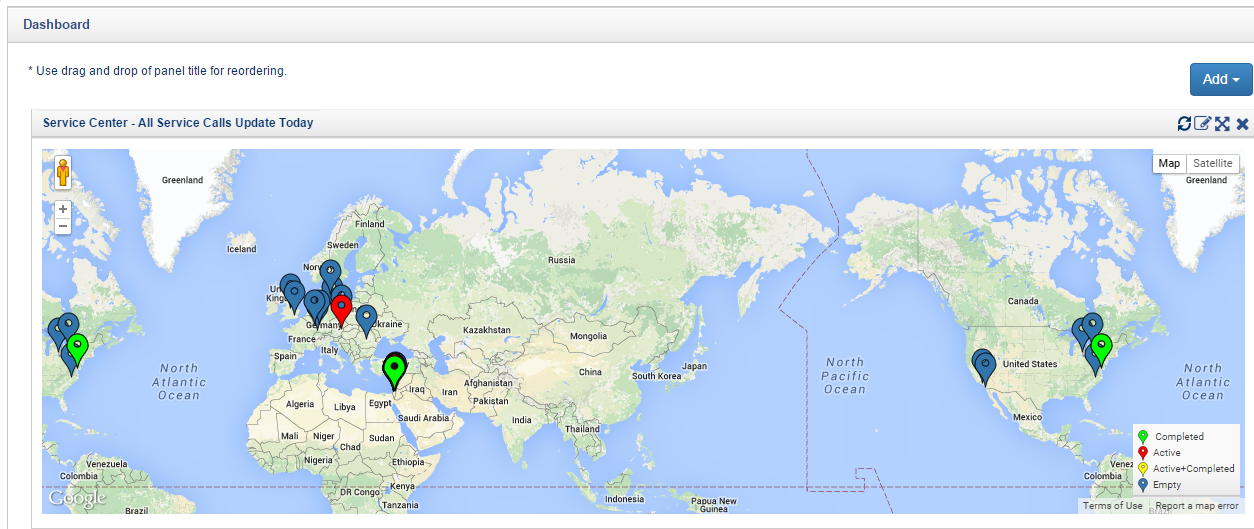
Fig 5: Follow Up Global Activity on Service Call using Orcanos Service Center
The tenth and final GMP principle is the need to continually audit the day-to-day job performance, and verify that a company is in compliance with the required GMP regulation.
For example, the FDA has a major responsibility to externally audit manufacturing operations to see if they are in compliance with the GMP regulation. However, it is a company’s responsibility to internally ensure the integrity of its products.
And, importantly, it is the personal responsibility of every employee to evaluate how well the company is living up to the standards of GMP. In fact, by performing a self-audit, using the 10 stated principles of GMP, good manufacturing practices become a daily lifestyle or habit, and not just one related to regulation.
It should be noted that in addition to a company’s responsibilities to its customers, entities such as the FDA have a responsibility to protect the consumer. As a result the FDA can recommend a recall if they find a product contaminated, mislabeled or not manufactured in compliance with the current GMP regulation.

Fig 6: Proactive Pre-Audit Reports/Alerts using Orcanos Reporting Tools
It is extremely important that companies and stakeholder carefully follow the 10 principles of GMP that I have outlined in this blog post. At our company, for instance, we are all concerned about what we do and how we do it. This concern for quality helps us earn the trust of millions of people who use our products. Ultimately, it is our job to make GMP a lifestyle for our customers, and that starts with living the principles themselves, each and every day.