Modern Quality Management for MedTech Teams
Orcanos assists medical device and pharma companies in streamlining compliance, removing silos, and speeding up time to market - all within one powerful platform.
✅ Built for ISO 13485, 21 CFR Part 11, EU-MDR
✅ Integrated Risk, CAPA, Design Controls
✅ Cloud-based, validated, audit-ready
Accelerate your process to achieve
ISO 13485 compliance
Orcanos QMS software provides a centralized platform to control all quality processes. This eliminates the need for a paper-based quality system and enables organizations to comply with strict FDA regulations and established ISO quality standards.
Designed for Regulated Teams
Built for Compliance. Designed for Speed!
Purpose-Built for Life Sciences
Manage ISO 13485, EU-MDR, and FDA 21 CFR compliance without spreadsheets or disconnected tools.
Audit-Ready at Every Step
Every document, signature, change, and CAPA is tracked and ready for review—no last-minute scrambles.
All-in-One Platform
Quality, risk, requirements, and design controls—unified in a single workspace.
Quality Management, Centered Around Your Product
Orcanos unifies quality management, training, and compliance in a single platform tightly integrated with your product design, risk, and requirement records. From concept to commercialization, ensure full traceability across your NPDI process—with built-in design control, automated workflows, and audit-ready documentation. One Platform. One Truth. Full Traceability.

Experience Audit-Ready Quality, Book Your Orcanos Demo Today!

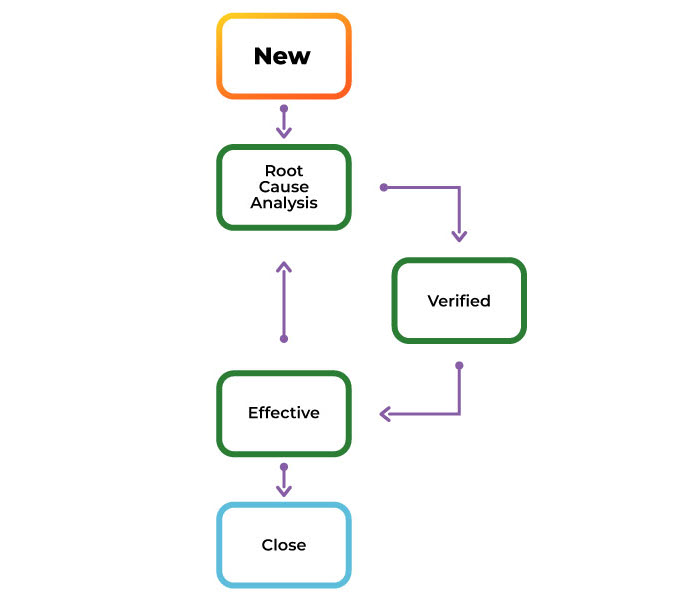
Streamline and Strengthen Your CAPA Process
Orcanos connects CAPA management with your product design, risk, and compliance records—so you can identify root causes, take action fast, and ensure traceable, audit-ready resolutions. From detection to closure, manage the full lifecycle of corrective and preventive actions in one unified system.
What our clients say about us

"Optimal solution with the right price and value"
We needed a better way to document and distribute requirements. Orcanos provided the optimal solution with the right price and value. As a startup, the attention from Orcanos helped us transition smoothly into the digital world. We're confident we made the right choice and look forward to growing with them.

"We obtained CE certification and FDA clearance with Orcanos."
We were impressed by Orcanos' attention and expertise in the medical device industry, and we felt it was the right choice for us. Having obtained both CE certification and FDA clearance with Orcanos, we are confident that we made the right choice for ZygoFix.
Take your regulatory compliance to the next level with Orcanos. Book a demo today and experience the power of our integrated solutions firsthand!

_HighPerformer_EMEA_HighPerformer.svg)
_FastestImplementation_Mid-Market_GoLiveTime.svg)

_HighPerformer_Mid-Market_EMEA_HighPerformer.svg)
.png)
.png)


































