Streamline Your Medical Device Quality & Development in One Platform
Orcanos adapts to YOUR way of working across R&D and Quality. Get up and running quickly with our eQMS best practice, available at any scale.
Our MedTech Software Suits

Next-Gen Medical Device eQMS
Built for Speed.
Audited for Excellence.
Go Live in 14 Days: Skip the 3-month implementation cycles of legacy providers.
Complete Traceability: Automatically link User Needs, Risks, and Validations.
Audit-Ready Always: Native support for ISO 13485:2026 and EU MDR.

Unified ALM & Design Control for Medical Devices
Accelerate your speed-to-market without compromising safety. Our integrated ALM platform bridges the gap between software development and regulated Design Controls. Ensure end-to-end traceability, automated risk management, and DHF audit readiness in a single, validated environment.
Bridge the Gap: One Source of Truth for QA and R&D.
When ALM and QMS live in silos, compliance fails. Orcanos bridges the gap with automated impact analysis and end-to-end traceability across product and Quality teams
Real-World Resilience: 4 Audits in 10 Days
A Customer Experience Story on the Power of Centralized eQMS.
"In the span of a week and a half, I had four days' worth of auditing... No back room. No binders. No scrambling for documents. Everything was handled directly inside Orcanos."
Hein Smit Sibinga, Director Quality & Regulatory at Covaris.
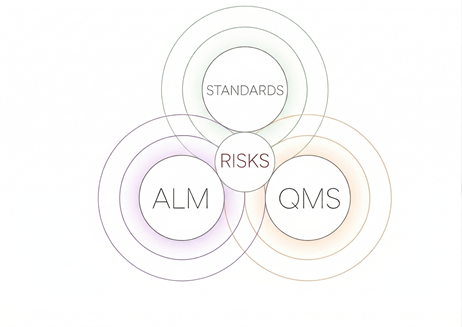
Accelerate MedTech Innovation by Bridging Development and Quality
Stop managing silos. Orcanos unifies ALM and QMS into a single source of truth, reducing compliance friction and cutting your time-to-market. Empower your teams to build faster without compromising on regulatory standards.
Read about Ask Paul, our new AI-powered assistant.
Transform your operations. Embrace innovation. Let Ask Paul guide you towards efficiency and excellence!

Orcanos Document Control: Eliminating Regulatory Friction
Manual document management drains engineering resources and stalls innovation. Orcanos solves the core bottlenecks that prevent Medical Device companies from staying audit-ready and agile
Automated Lifecycle: From flexible workflow routing to automated PDF publishing and archiving.
Absolute Traceability: A centralized, 21 CFR Part 11 compliant repository with a detailed audit trail for every revision.
Training Automation: Automated "Read and Understad" tasks fired instantly upon document release to ensure the team is always up-to-date.


Adaptive Compliance: Your Process, Your Rules
Tired of being forced to change your workflow to adapt to your QMS? Most systems demand you work "their way." We believe your QMS should adapt to you, not the other way around.
Orcanos Risk Management: Beyond ISO 14971 Compliance
The Challenge Static Excel sheets create "disconnected truth"—manual risk files are outdated the moment they’re saved, leading to traceability gaps and audit failuresThe Orcanos Solution:We turn risk into a live, integrated engine. By unifying ISO 14971 with your ALM and QMS, Orcanos automates the "Excel tax," cutting manual management costs by 30% while ensuring your risk profile evolves instantly with every design change.
Live Traceability:Automatic links from requirements to hazards to mitigations.Visual Certainty:Real-time heatmaps for instant visibility into critical safety trends.
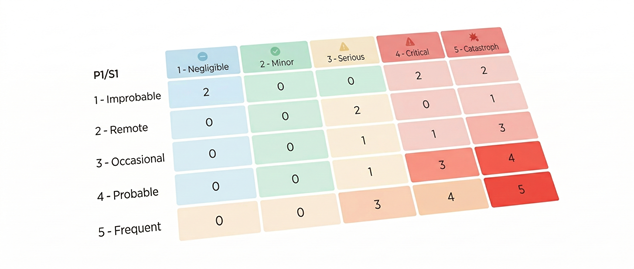
What our clients say about us

"Optimal solution with the right price and value"
We needed a better way to document and distribute requirements. Orcanos provided the optimal solution with the right price and value. As a startup, the attention from Orcanos helped us transition smoothly into the digital world. We're confident we made the right choice and look forward to growing with them.

"We obtained CE certification and FDA clearance with Orcanos."
We were impressed by Orcanos' attention and expertise in the medical device industry, and we felt it was the right choice for us. Having obtained both CE certification and FDA clearance with Orcanos, we are confident that we made the right choice for ZygoFix.
Why Medical Device Teams Choose Orcanos
Stop Fighting Documentation, Start Driving Discovery
For medical device innovators, the burden of regulatory compliance often feels like a brake on innovation. Orcanos removes that friction by uniting Design Control, QMS, and Risk into a single, automated source of truth.
Continous Audit Readines
The Challenge:Scrambling to meet FDA, MDR, and global requirements during surprise audits.The Solution: Built with compliance at the core, we provide total peace of mind that your data is always audit-ready.
Accelerated Time-to-Market
The Challenge:Bottlenecks in documentation and manual approval workflows that delay product launches.The Solution:Automate compliance processes and streamline workflows to shift focus from paperwork back to discovery.
Deep Traceability: From Requirement to Risk
The Challenge: Gaps in your traceability matrix that only surface during a regulatory review, leading to costly delays.The Solution: Orcanos threads ALM and QMS together, ensuring that every design change is automatically assessed for risk (ISO 14971) and regulatory impact.
The "One Source of Truth" Efficiency
The Challeneg: Data silos where R&D, Quality, and Operations work from different versions of the truth, leading to nonconformances.The Solution: One unified platform that serves as a centralized repository. This eliminates the "documentation tax," allowing your engineers to focus on discovery, not paperwork.
Rapid Deployment & Customization
The Challenge: Rigid "out-of-the-box" software that forces you to change your validated SOPs to fit their tool.The Solution: Powerful customization tools allow us to tailor the platform to your specific SOPs, status flows, and permission levels, ensuring the software works for your team, not against them.
Regulatory Confidence Built Into the Workflow
The Challeneg: Relying on human memory to follow regulaiton guidelines and protocols.The Solution:Our platform enforces compliance. Electronic signatures, mandatory fields, and status flows are baked into the system, making "doing it right" the only way to do it.
Take your regulatory compliance to the next level with Orcanos. Book a demo today and experience the power of our integrated solutions firsthand!

_HighPerformer_EMEA_HighPerformer.svg)
_FastestImplementation_Mid-Market_GoLiveTime.svg)

_HighPerformer_Mid-Market_EMEA_HighPerformer.svg)
.png)
.png)



































