
Overview
A traceability matrix is a cornerstone of design control compliance for medical device manufacturers. ISO 13485 and FDA 21 CFR Part 820.30 require establishing clear links between Design Inputs, Design Outputs, Verification & Validation (V&V), and Risk Controls.
Companies risk audit findings, missed requirements, and regulatory noncompliance without a centralized, accurate traceability system.
Migrating from Excel-based traceability matrices to the Orcanos eQMS platform is a major step toward better product lifecycle management. While the transition may appear to reduce the number of traceable items, it actually results in: clearer, more maintainable and meaningful traceability structures. Avoiding unnecessary over-tracing and redundant links between requirements, tests, and risks.
Understanding the V-MODEL in Medical Device Design Control
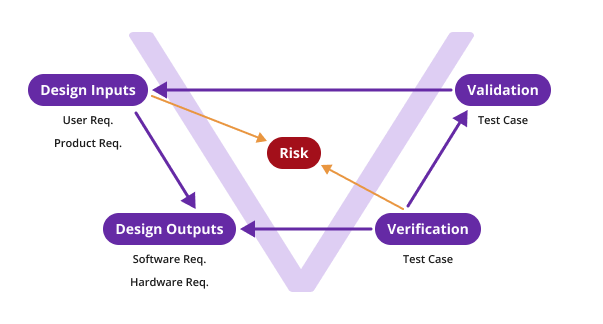
To align with industry best practices and regulatory expectations, Orcanos supports the widely recognized V-model of design and development.
This model is a common framework in the medical device industry and demonstrates how design inputs are translated into and implemented through design outputs. These outputs are verified and validated against the original inputs, with risk management activities occurring across the lifecycle. This framework is the foundation for traceability in Orcanos, helping ensure that every requirement and risk is addressed through appropriate testing and controls.
Below is a visual representation of this model, highlighting the relationship between requirements, risk, and testing across the development process:
How Orcanos Simplifies Traceability Compared to Excel Sheets
Many organizations using Excel for traceability fall into the trap of creating unnecessarily complex Level 4 or Level 5 traceability matrices—where every granular link (e.g., user need → system requirement → software requirement → design detail → test case) is explicitly traced. While this may seem comprehensive, it often results in:
❌ Over-tracing and redundant relationships
❌ High maintenance overhead for each trace level
❌ Manual errors and outdated links
❌ Poor readability and usability during audits
❌ Difficulty in change impact analysis
❌ Wasted effort on non-regulatory-driven trace levels
Orcanos solves this by focusing on value-driven traceability that aligns with ISO 13485 and FDA expectations—without overburdening teams.
.png)
Instead of tracking every low-level engineering detail across five columns, Orcanos empowers teams to focus on what matters: verifying that design outputs meet inputs, validating that user needs are fulfilled, and ensuring risks are mitigated—all traceable from a single platform.
This results in a system that’s easier to manage, easier to audit, and more compliant, with less room for error and rework.
Orcanos Traceability Matrix Options
All-in-One Traceability Matrix
The default Orcanos traceability matrix provides a comprehensive view that connects all regulatory-required traceability relationships in a single table:
.png)
This centralized view is ideal for demonstrating full traceability during audits and for performing impact assessments when requirements or risks change.
Optional Breakdown: Separate Verification and Validation Views
For teams or auditors who prefer a more structured approach, Orcanos allows the traceability matrix to be split into dedicated views for verification and validation activities.
This flexibility helps meet different organizational and regulatory reporting preferences—without duplicating data or creating unnecessary complexity.
Validation
Maps design inputs (high-level requirements) to their respective validation test cases, ensuring the final product fulfills its intended use.
.png)
Verification
Connects design outputs (low-level requirements) to their respective verification test cases, ensuring that each implemented feature functions as intended.
.png)
Conclusion
With Orcanos, simplifying traceability isn’t just about eliminating complexity—it’s about empowering teams to focus on compliance, product quality, and innovation without manual overload.Traceability doesn’t have to be messy or overwhelming. With a clear structure and the right tools, it becomes a natural part of your workflow—not a burden. Moving from Excel to Orcanos isn’t just about compliance—it’s about making life easier for everyone involved.A well-structured traceability matrix doesn’t just satisfy auditors—it supports better decisions, improves communication across teams, and ensures that nothing critical is overlooked. As regulatory expectations grow more complex, having a system that brings everything together in one place isn’t a luxury—it’s essential.
If you would like a personalized demo please schedule here.




.png)

































