Test Management Tools
Streamline your medical device testing process with our powerful Test Management Tool - Ensure Compliance, Traceability and Product Safety.


Orcanos Test Management Tool Featuring
Test Execution
Efficiently manage test cases with Orcanos. Plan, execute, and record results while tracking progress using an intuitive dashboard.
Report defects directly from test runs with an integrated bug tracking tool.
When reporting a defect, all relevant test case information, including steps, is automatically imported into the new bug.
Mark tests as Pass, Fail, or Blocker easily.

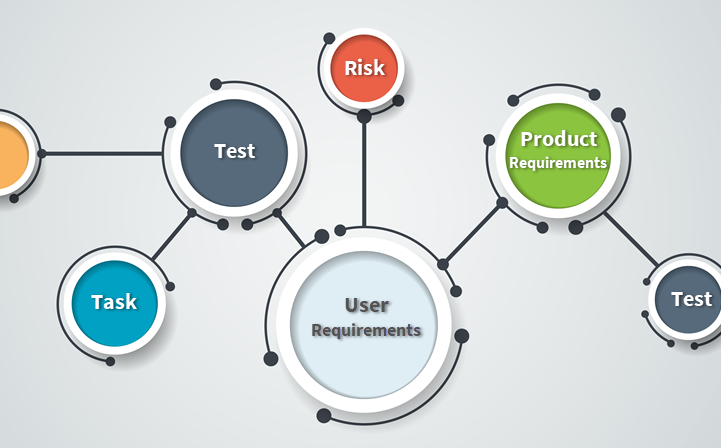
Traceability and Requirements Coverage
The Orcanos testing system is tightly integrated into ALM modules, such as requirements management and FMEA risk management. this integration ensures full traceability of all ALM artifacts in a single repository.
Medical Device Testing Process Simple. Traceable, Simple, Automated, Call us for more.

Additional Features
Risk Control
Integrate Design Control (ALM) with Quality Management System and regulation to establish a centralized repository.
Risk Verfication
test cases can be tracked and traced to ensure that the level of risk has been properly assessed and verified, with these results saved for potential verification evidence.
Easy customization
Save 30% of the resources spent on manual management and integrate risk into your development, and your QMS processes, assuring full traceability.
Test Parameters
Use test parameters to build predefined configurations, and use them while executing the test.
Test Tracking and Reports
Comprehensive reports and graphs for enhanced test plan control and governance.


Word Document Generation with One Click
A perfect match for medical device manufacturers who are looking for a way to cut hours and resources for preparing documents for submission.


































